
home > research > dipolar EPR on membrane transport complexes
Dipolar EPR Spectroscopy on Membrane Transport Complexes
project within SFB 807: Transport and Communication across Biological MembranesIn this project pulsed electron paramagnetic resonance spectroscopy is used to investigate structural changes and conformational flexibility of membrane transport proteins. We aim to trap the transport process in different functional states or changes induced by the lipid environment. Pulsed Electron-Electron Double Resonance (PELDOR) spectroscopy will be used to determine long-range distance constraints in the nm range on site-specific spin-labeled proteins or protein complexes.
We applied this spectroscopic method to membrane transporters (such as LmrA (Fig. 1)[1] and lysosomal polypeptide transporter TAPL) as well as ion channels (such as KcsA, KvAP) in specific functional states to obtain information about conformational changes related to the transport function, oligomerization state and substrate binding.
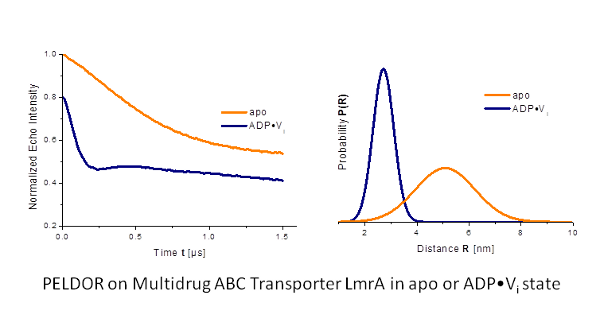
Our goal is to study membrane proteins in lipid membranes. However, the transversal relaxation time (Tm) is often very short in such environments.
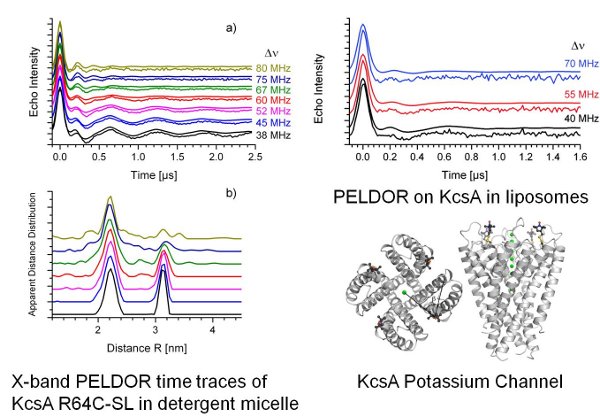
We did optimize the sample conditions for PELDOR on proteins in membranes and achieved Tm values of up to 5µs.[2]
We successfully applied the optimization
procedure on the KcsA potassium channel in liposomes (Fig. 2). [3]
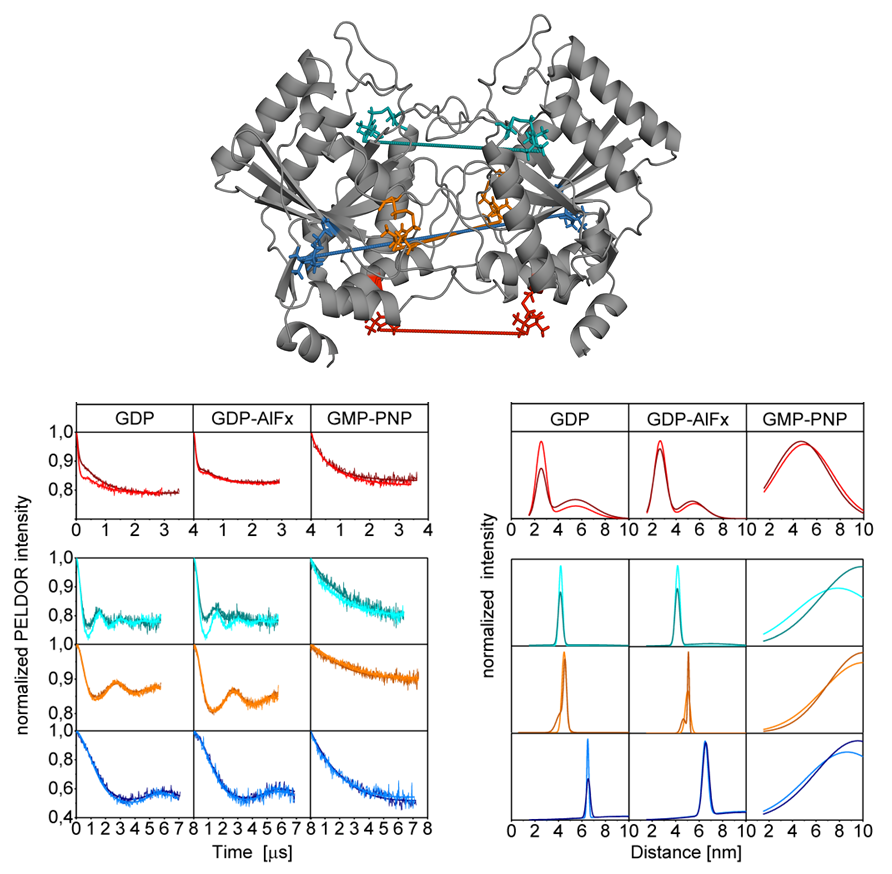
One of our (P7 Prisner) joint projects within the SFB 807 "Transport and Communication across Biological Membranes" comprises the investigation of the homodimeric psToc34 and the heterodimeric psToc34-psToc159 G GTPase domains from Pisum sativum in different functional states by PELDOR spectroscopy [4]. (collaboration with P17 Schleiff), (Fig. 3)
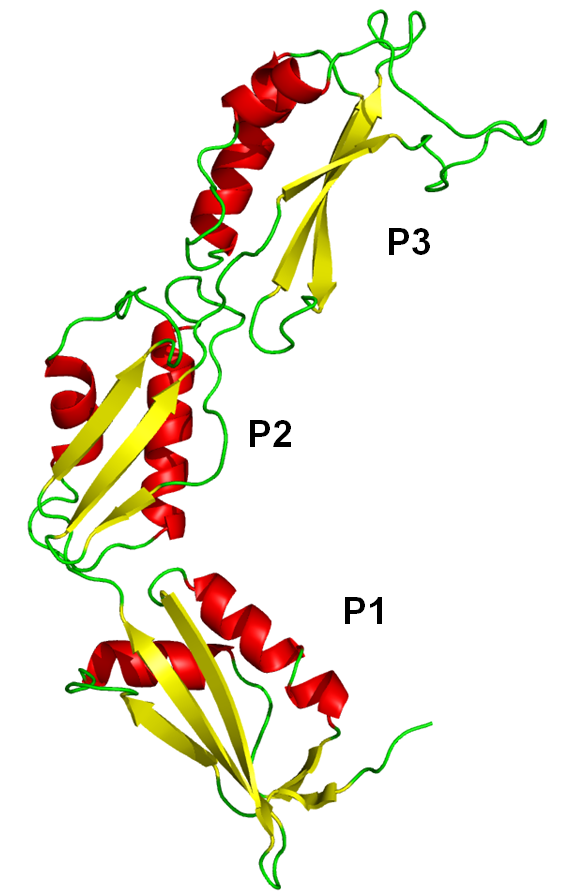
Within the joint project, in collaboration with P17 Schleiff, we use site-directed spin labeling (SDSL) to investigate the conformational flexibility between N-terminal POTRA domains of Omp85 from the cyanobacterium Anabaena sp. PCC 7120 (Fig. 4).
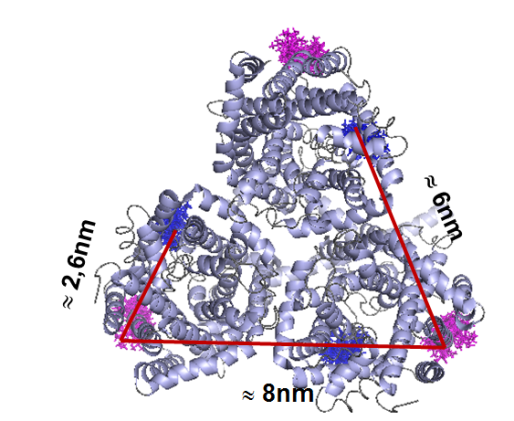
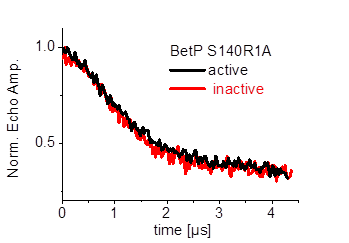
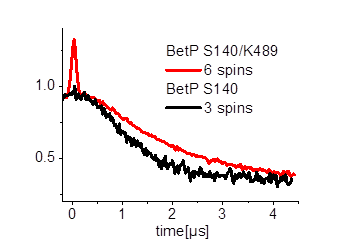
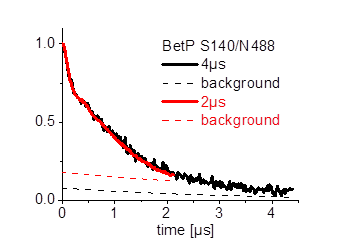
Furthermore we are investigating the trimeric betaine symporter BetP of C. glutamicum (collaboration with P4 Ziegler). BetP is a member of the family betaine/ choline/ carnitine transporters (BCCTs) and has three different functions: catalytic activity of betaine transport, sensing of osmotic stress, osmoregulation (adjusting transport rate with stress extent). In our studies we use PELDOR spectroscopy to characterize the structural changes of functional states within the BetP transport cycle. (Fig. 5)
Other projects are concerning the photoactive retinal protein proteorhodopsin (collaboration with P6 Glaubitz and P12 Wachtveitl) and the TMD0 domain structure and its interaction with TAP1 and TAP2 (collaboration with P16 Tampé and P19 Schäfer).
References:
[1] U. A. Hellmich, et al., J. Am. Chem. Soc. 2012, 134, 5857.
[2] R. Dastvan, et al., J. Phys. Chem. B 2010, 114, 13507.
[3] B. Endeward, et al., J. Am. Chem. Soc. 2009, 131, 15246.
[4] C. Lumme, et al., Structure, 2014, 22, 526.
| Last modified: April 24, 2019 | Impressum / About / Legal Notice | Datenschutzerklärung |
Prisner LOGS
Webmail
Internal
|